Best method for learning Periodic Table?
Date-28/09/20
Hello Dear Friends, Today In this article about Modern Periodic Table. I describes this topic with fully informative and like there uses in Chemistry. I recall when you was studying in class 9th to 12th, their uses you have to know. So I am describing about this topic. Lets start……
( 1. What is Periodic Table?
· In Periodic table, a group of elements (like, metals, non-metals, metalloids or gases) arrangement with their atomic properties in a chart is known as Periodic Table.
( 2. How many Elements in Modern Periodic Table or Periods?
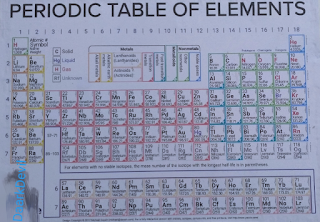
· In this Modern Periodic Table, the number of elements is 118 in which all elements are arranged in Periods and Groups. The number of Periods and Groups is 7 Periods or 18 Groups. The elements are divided in 4 Blocks, which are S-Block, P-Block, D-Block and F-Block. A picture is given below see this image carefully.
( 3. How many Metals, Non-metals and Metalloids in Modern Periodic Table?
· In Periodic Table, Metals are around 95 of 118 elements, Non-metals are around 17 elements of 118 elements, and Metalloids are around 6 of 118 elements. You can add the numbers for satisfying 95 metals + 17 non-metals + 6 metalloids =118 elements.
( 4. How to learn Periodic Table and properties of groups or Periods?
Here are the best method for learning periodic table:-
· In S-Block of 1st Group, this group is known as Alkali Metals, you can learn like this { HeLiNaKiRabCsFariyar } in hindi { हेलिना की रब से फरियार }
S-Block (1st
Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
H |
1 |
Hydrogen |
|
Li |
3 |
Lithium |
|
Na |
11 |
Sodium |
|
K |
19 |
Potassium |
|
Rb |
37 |
Rubidium |
|
Cs |
55 |
Cesium |
|
Fr |
87 |
Francium |
S-Block
(2nd Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
Be |
4 |
Beryllium |
|
Mg |
12 |
Magnesium |
|
Ca |
20 |
Calcium |
|
Sr |
38 |
Strontium |
|
Ba |
56 |
Barium |
|
Ra |
88 |
Radium |
P-Block
(13th Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
B |
5 |
Boron |
|
Al |
13 |
Aluminium |
|
Ga |
31 |
Gallium |
|
In |
49 |
Indium |
|
Tl |
81 |
Thallium |
P-Block
(14th Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
C |
6 |
Carbon |
|
Si |
14 |
Silicon |
|
Ge |
32 |
Germanium |
|
Sn |
50 |
Tin |
|
Pb |
82 |
Lead |
P-Block
(15th Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
N |
7 |
Nitrogen |
|
P |
15 |
Phosphorus |
|
As |
33 |
Arsenic |
|
Sb |
51 |
Antimony |
|
Bi |
83 |
Bismuth |
P-Block
(16th Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
O |
8 |
Oxygen |
|
S |
16 |
Sulphur |
|
Se |
34 |
Selenium |
|
Te |
52 |
Tellurium |
|
Po |
84 |
Polonium |
· In P-Block of 17th Group, this group is known as Halogens,
learn like this { Fhir Cal Bahar I Actress
} read this { फिर कल बाहर आई एक्ट्रेस }
P-Block
(17th Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
F |
9 |
Fluorine |
|
Cl |
17 |
Chlorine |
|
Br |
35 |
Bromine |
|
I |
53 |
Iodine |
|
At |
85 |
Astatine |
· In P-Block of 18th Group, this group is known as Noble
Gases, you can learn this { He Ne Ar Kor Xe Ren }
read this as { ही ने आर कोर एक्स रेन }
P-Block
(18th Group)
|
Symbol |
Atomic No. |
Atomic Name |
|
He |
2 |
Helium |
|
Ne |
10 |
Neon |
|
Ar |
18 |
Argon |
|
Kr |
36 |
Krypton |
|
Xe |
54 |
Xenon |
|
Rn |
86 |
Radon |
I hope You Understand--Thank You! For any Questions
Please Comment.
![Daark Devil [Age of Technology]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEgSnBiStRJzy89ezUpAF0FWB7D2onPqMjhV-8HM6A3IAmaaKXidX1Myq3oVs5VXyXsPr48ii63opzWiz94nzn7hsRsdxMK_yHT8wctg3-fIspS81vOQogsuJgxmZTNRSGlBuCjaJpOl2pU/s1600/DaarkDevil11.png)







16 Comments
Bahut badia Gyan dia h apne
ReplyDeleteAmazing chemistry Knowledge.. bro...
ReplyDeletenice info ...
ReplyDeleteBuaya Terbang Blog
Amazing method bro!!!!
ReplyDeleteNice knowledgeable Article
ReplyDeleteGood
ReplyDeleteVery Informatic
ReplyDeleteThanks for information
ReplyDeleteGreat post!
ReplyDeleteAmazing 👍
ReplyDeleteNice one 👍
ReplyDeleteGood nice post hanks for sharing keep it up Brother
ReplyDeleteNice post,,,
ReplyDeleteAmezing buddy. Good work done
ReplyDeleteAwesome trick keep it up brother
ReplyDeletethe grumbles were again about the abuse of PowerPoint's and the way that teachers use it to discuss what's on the scale. Another grumbling was that instructors who are new to innovation frequently burn through class time as they invest more energy investigating than educating. Oracle Procurement Training
ReplyDelete